[Experimental operation and phenomena]
Brown's demonstration
(1) Put about 300 steel balls into the simulation box, so that the steel balls are roughly covered with the bottom layer of the box, and then the Brown particles (foamed plastic blocks) are placed. At this time, the floating piston is inserted and covered. A rubber plug with a hole is arranged on the piston rod, and the floating piston is adjusted and fixed at a certain height (generally at a height of 10-15 cm).
(2) When the power is turned on, the vibrator balls are chaotically moved under the impact of the vibrating plates (the vibrators also collide with each other). Adjusting the voltage can make the vibrator chaotic movement change drastically. At this time, it can be seen that the Brownian particles continuously move under the collision of the steel balls (molecules). Due to the irregular shape of the Brownian particles, it can be seen in the experiment that the Brownian particles move sideways and form a distinct Brownian motion.
2. Demonstration of statistical significance of gas pressure
(1) Experimental principle
Theoretical studies have shown that the gas pressure can be determined by the impulse change caused by the collision of a molecule on a certain surface (such as the wall), and the molecular collision wall at each moment, the magnitude of the impulse transmitted to the wall during the collision, is accidental, thus reflecting The statistical average (gas pressure) of a large number of molecular collision impulses must fluctuate. The size of the undulation is related to the number of molecules (the undulation is proportional to 1/n). When the molecular density is n hours, the undulation is large; when the molecular density n is large, the undulation is small. This statistical significance of gas pressure can be demonstrated using molecular motion theory demonstrators.
(2) Experimental operation
Install dozens of vibrators in the square box. At this time, the pressure value caused by the vibration of a small number of vibrators is very unstable. In the experiment, we see that the floating pistons are up and down undulating; when a large number of vibrators are placed in the square box, Seeing that there is a stable pressure value, the floating piston is in a certain position under the impact of a large number of vibrators, and the undulation is small.
3. Demonstration of the ideal gas state equation
Using the assumptions of the molecular motion theory on the ideal gas model, the equation of state of the ideal gas can be derived, ie
or
(74-1)
For a certain quality (
Certain or total number of molecules
a certain ideal gas, from its equation of state, when the temperature
Certain time, gas pressure
Molecular density
In direct proportion.
Put about 300 particles in the simulation box, that is, the total number of molecules N = 300, as shown in Figure 74-2. In the case of the rated number of revolutions of the motor, due to the chaotic movement of the vibrator due to the impact of the vibrating plate, a certain pressure is generated (by the weight of the floating piston)
Said), then the floating piston can be measured
Position; this is equivalent to the guaranteed temperature when the rated number of revolutions of the motor is constant
Constant (that is, the vibration of the vibrator caused by the intensity is constant), the weight of the movable piston is doubled with the prepared weight, and the pressure becomes
, the floating piston can be measured
Position; when the pressure is
When measured, the volume is
.
It can be seen that when the pressure is
Corresponding molecular density
When the pressure is
Time,
When the pressure is
It can be seen that when the temperature is constant (the motor speed is constant), the pressure is proportional to the particle number density, ie
.
4. Demonstration of the actual gas state equation
(1) Experimental principle
For the steel ball model, the actual gas state equation can be written as:
(74-2)
Where is the gas quality,
For the molar mass of the gas,
For a correction value,
It is the volume of the steel ball oscillator itself. This demonstrator can be rated at the rated power of the motor (corresponding to temperature
Certainly) obtained through experiments
To demonstrate the actual gas status agenda.
(2) Demonstration method
A layer of steel ball vibrator (300 particles) is placed in the simulation box to cause the motor to move at a certain rated speed, causing the floating piston to be in the A position, as shown in Figure 74-2. To ensure that the rated speed of the motor is constant, the floating piston is doubled by the preparation weight, and the piston reaches the B position. Considered by ideal gas at temperature
And the number of particles
Under certain circumstances, the product of gas pressure and volume is a constant, ie pV=constant
The corresponding situation in this experiment is
then
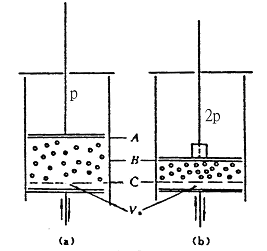
Figure 74-2
and
Therefore
According to the above calculation, the volume above the C line in the figure is the volume of the ideal gas molecule activity, and the volume below the C line to the vibration plate (centered position).
This is the volume correction caused by the actual gas molecule (steel ball oscillator) itself having a certain volume. The size of the experiment is measured, and then with the steel ball vibrator (
Grain) itself
In comparison, the calculation results show that:
(74-3)
It can be seen that considering the influence of the volume of the molecule itself, the actual gas molecule activity volume should be
This demonstrates a state equation for a gas.
5. Demonstration of the Boltzmann distribution law
(1) Experimental principle
In the gravitational field, the number density of ideal gas molecules obeys the Boltzmann distribution law by height, ie
(74-4)
(2) Demonstration method:
1) Insert 300 pieces (or more) of the vibrator in the simulation box, cover the cover plate, and insert the end of the molecular number separation compartment (multi-layer insert) into the box baffle with the gap.
2) Adjust the voltage (generally not too large, such as 5V) to make the vibrator vibrate. At this time, a large number of vibrators form a certain distribution according to the height in the gravity field. After a short period of time, quickly insert the multi-layer insert into the simulation box by hand, and the vibrator is separated into different high-rises by the separation slot. Turn off the power.
3) Tilt the square box to the right, and the vibrator balls of each layer are arranged in the respective compartments.
Observing the number of particles in each layer, drawing a curve from the first layer of the cell, it can be seen that the curve decays with the negative exponential of height, thus demonstrating the Boltzmann distribution law.
Adjustable Single Desk And Chair
Adjustable Single Desk And Chair,Desks And Chairs,School Study Chair Table,Adjustable Kid Desk And Chair
AU-PINY FURNITURE CO., LTD , https://www.jmaupiny.com
